Abstract
Autologous stem cell transplant (ASCT) involves high intensity conditioning regimens with significant cytotoxicity. Therapy-related myeloid neoplasms (tMN) represent a serious complication characterized by high-risk features and poor treatment outcomes in patients potentially cured from their primary neoplastic disorders. While the 2016 WHO classification broadly divides tMN into MDS or AML, yet their heterogeneity warrants a more distinctive characterization based on specific prior exposures and subsequently-developed unique features, as emphasized by the updated 2022 WHO revision. We previously analyzed the genomics of tMN including features suggestive of their derivation from either CHIP or de novo driver mutations1. Specifically for tMN pathobiology, ASCT may be very instructive due to the high dose therapy and repopulation of the hematopoiesis out of a limited number of transplanted hematopoietic stem cells. Post-ASCT tMN may be unique and possibly related to antecedent CHIP, CHIP induced by the procedure, or driver lesions. We took advantage of a large volume of ASCT performed at our institution along with excellent clinical annotations and long follow up to study the dynamics of tMN in this setting.
We accrued a total of 1507 patients undergone ASCT, of whom 35 (2.3%) developed tMN at a median follow up time of 5.4 years following the procedure. The clinical and molecular phenotype of these index cases were then compared to a cohort of tMN patients (n=263) without prior history of ASCT. Of note, both groups had similar MDS/AML proportions.
Compared to the rest of the ASCT cohort, patients developing tMN were older at the time of the transplant procedure (66 vs 46% ≥60 years, P=.02) and had a higher male preponderance (91 vs 59%, P<.0001). Post-ASCT tMN was more common following non-Hodgkin's lymphoma (NHL) than multiple myeloma (MM) (2.5 vs 1.1%, P=.0002) but similar between NHL and Hodgkin's disease (2.5 vs 1.5%, P=0.13). We then studied whether graft size and mobilization regimens could increase the risk of tMN. The incidence of this complications was higher with a mobilization involving GCSF (G), Etoposide (E) and Plerixafor (P) (GEP) rather than G, G+E or G+P (P=.0001), as well as when a CD34+ dose ≤3.5 x106 (4.1 vs 1.9%, P=.03) was infused. No difference was noted with respect to time to engraftment following ASCT. Multivariate analysis confirmed the independent influence of age ≥60 (HR 2.4, 95% CI 1.1-5.6), male sex (HR 7.1, 2.5-29.9), diagnosis (prior NHL vs MM; HR 3.3, 1.5-8.2) and mobilization scheme (GEP vs G+P; HR 4.7, 1.3-13.7). The caveat was the lack of significant effect of the graft size on tMN risk.
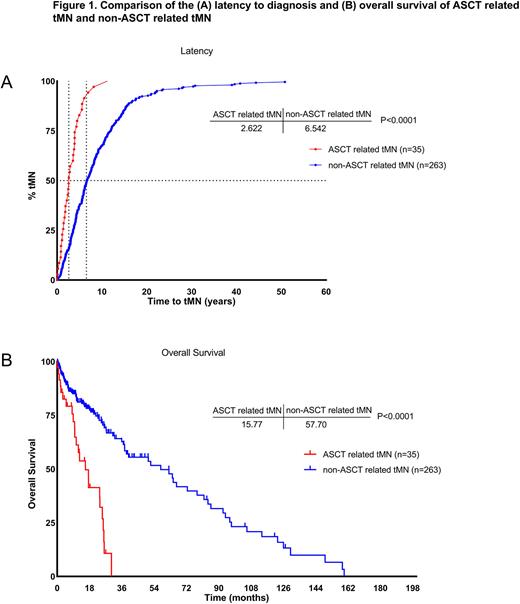
When compared to non-transplanted cases, tMN following ASCT still showed a male preponderance (91 vs 51%, P<.0001) and exhibited a higher prevalence of high-risk (IPSS-R>3.5) MDS (79 vs 57%, P=.05). Furthermore, tMN arising post-ASCT appeared to follow a more aggressive course with a significantly shorter latency duration following the procedure (median 2.6 vs 6.5 years, P<.0001) and shorter overall survival (median 16 vs 58 months, P<.0001; Fig1). Cytogenetic profiling revealed higher frequencies of complex karyotype (49 vs 31%, P=.05), del(5q)/5- (26 vs 20%, P=.5), del(7q)/7- (46 vs 27.5%, P=.03), and del(17p)/17- (27 vs 6%, P=.0025) in the ASCT-exposed as opposed to non-transplanted cases. Subsequent analysis of isolated cytogenetic aberrations showed that isolated del(7q)/7- was significantly enriched in post-ASCT tMN (20 vs 6%, P=.0129). Mutational analysis revealed similar patterns with the exception of TP53, which was significantly more frequent in the ASCT-exposed group (41 vs 13%, P=.0028), mostly displaying a multi-hit configuration (32 vs 11%, P=.0178).
We have previously shown that ancestral hits in DNMT3A, TET2, ASXL1 and JAK2 (CHIP-mutations) indicate derivation of disease from CHIP vsU2AF1, RUNX1 and STAG2 highlighted de novo cases, thereby prompting us to investigate whether CHIP may have been the originator of tMN post ASCT2. However, the frequencies of CHIP- and de novo -mutations were similar across the post-ASCT tMN and the non-ASCT tMN groups. Interestingly, within the post-ASCT tMN, patients who had CHIP-derived tMN had lower survival compared to the rest of the group (median 9 vs 25 months, P=.0079).
Sequencing experiments on serial specimens are ongoing to determine whether CHIP- derived mutations were present prior to ASCT constituting a risk for tMN.
Disclosures
Maciejewski:Alexion: Consultancy; Apellis Pharmaceuticals: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.